isotope
Jump to navigation
Jump to search
See also: Isotope
English
[edit]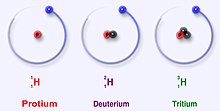
Etymology 1
[edit]From iso- (“equal”) + -tope (“place”), because the different isotopes of a chemical element always occupy the same position in the periodic table of elements. The term was coined by Scottish doctor Margaret Todd in 1909 and first used publicly on February 27, 1913 by English chemist Frederick Soddy.
Pronunciation
[edit]- (Received Pronunciation) IPA(key): /ˈaɪ.sə.təʊp/
- (US) enPR: ī'sətōp, IPA(key): /ˈaɪ.sə.toʊp/
Audio (US): (file)
Noun
[edit]isotope (plural isotopes)
- (nuclear physics) Any of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei. Thus, isotopes have the same atomic number but a different mass number.
Usage notes
[edit]Technically, isotopes are nuclides having the same atomic number but different mass number. In practice, the term isotope is often used instead of nuclide.
Derived terms
[edit]Translations
[edit]atoms of the same element having a different number of neutrons
|
See also
[edit]Etymology 2
[edit]Possible back-formation from isotopy.
Pronunciation
[edit]Verb
[edit]isotope (third-person singular simple present isotopes, present participle isotoping, simple past and past participle isotoped)
- (topology, transitive) To define or demonstrate an isotopy of (one map with another).
Related terms
[edit]Anagrams
[edit]French
[edit]Pronunciation
[edit]Adjective
[edit]isotope (plural isotopes)
Noun
[edit]isotope m (plural isotopes)
Descendants
[edit]Further reading
[edit]- “isotope”, in Trésor de la langue française informatisé [Digitized Treasury of the French Language], 2012.
German
[edit]Adjective
[edit]isotope
- inflection of isotop:
Latin
[edit]Noun
[edit]isotope
Categories:
- English terms prefixed with iso-
- English terms suffixed with -tope
- English coinages
- English 3-syllable words
- English terms with IPA pronunciation
- English terms with audio links
- English lemmas
- English nouns
- English countable nouns
- en:Nuclear physics
- English verbs
- en:Topology
- English transitive verbs
- en:Isotopes
- en:Neutron
- English back-formations
- French 3-syllable words
- French terms with IPA pronunciation
- French terms with audio links
- French lemmas
- French adjectives
- French relational adjectives
- French nouns
- French countable nouns
- French masculine nouns
- German non-lemma forms
- German adjective forms
- Latin non-lemma forms
- Latin noun forms
